Welcome to the official website mall of Shanghai Rainminda Pharmaceutical Technology Co., Ltd
EN
Welcome to the official website mall of Shanghai Rainminda Pharmaceutical Technology Co., Ltd
Virus Carrier CDMO Service
One-stop service for multiple virus vectors
We offer a one-stop CDMO service for the development of HSV、ADV、AAV、NDV、EV71、CV21、Vaccinia Virus、Lentivirus 、Retrovirus A one-stop CDMO service for virus vector development, our service cover the entire chain of concept validation, process development, pilot production, IND application, and clinical trials.
Herpes simplex virus(HSV):Oncolytic virus, gene therapy delivery vector. Large insertable gene fragments with good safety.
Newcastle disease virus(NDV):Oncolytic virus, gene therapy delivery vector.
Adenovirus(ADV):Oncolytic viruses and gene therapy delivery vectors with strong in vivo diffusion capabilities.
Adeno-Associated Virus (AAV):Gene therapy delivery vectors have extremely low immunogenicity and good safety.
Poxvirus:Oncolytic virus, with a large genome and a large capacity for inserting exogenous genes, has good safety.
Other viurs:Coxsackie virus, lentivirus, retrovirus, and other viral vectors can be developed as oncolytic viruses or gene therapy vectors.
Characteristics of Viral Vector Platform Services
A comprehensive quality management system:a quality management system and robust documentation based on ICH/NMPA/FDA/EMA/WHO standards, with complete original records and clear material traceability.
Fast CMC:The CMC team has extensive experience and can shorten project timelines by up to 6 months. The fastest timeline from DNA to IND submission is 12 months.
Multiple successful projects reported by both China and the United States:Several projects have successfully obtained IND approvals from China and the United States. The equipment and facilities are well-equipped, with multiple Full GMP and GMP like workshops available for selection.
The entire process of the innovative drug development:Mastery of the entire process of the innovative drug development including: drug target screening, preclinical efficacy, pharmaceutical research, quality research, pilot production, IND application, and clinical trials, aiding clients in the rapidly developing of the innovative drugs in various aspects.
Process flow of virus vector platform
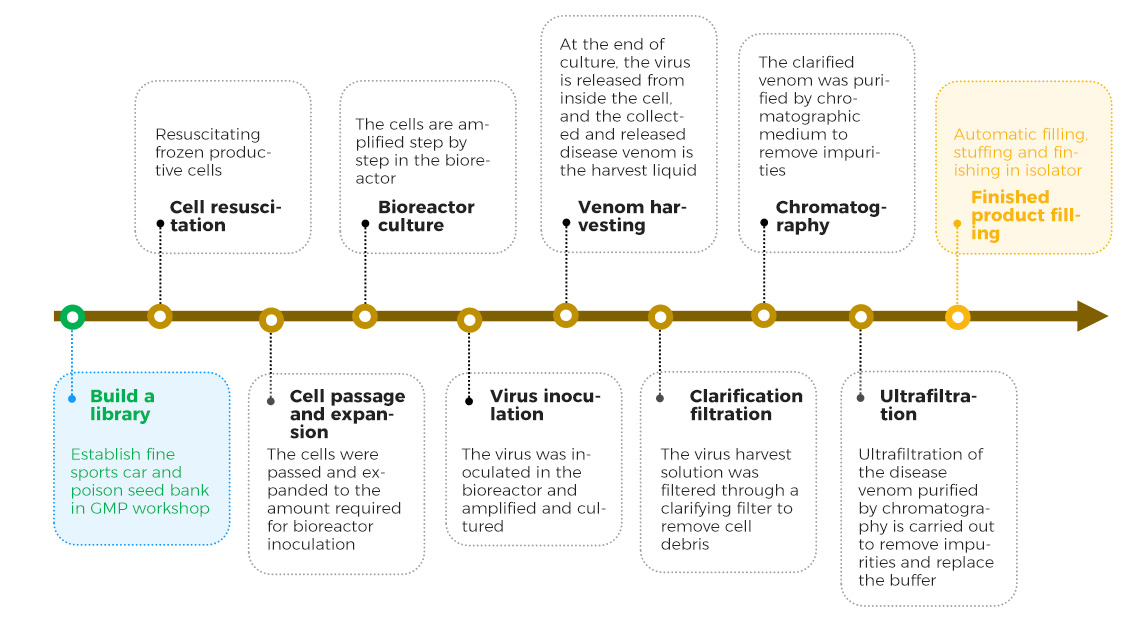
Process development strategy of virus vector platform based on QbD concept
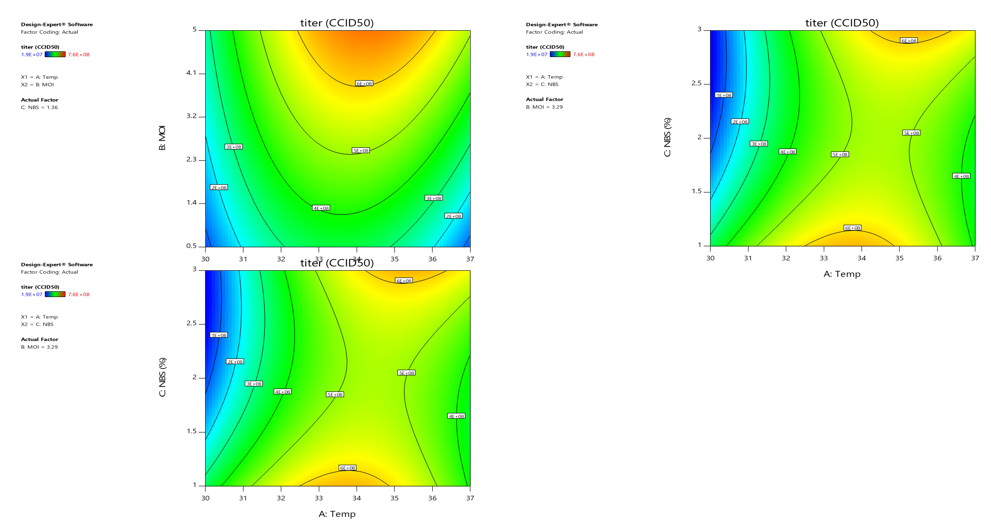
Process development is based on the QbD principle
Using DOE in process development to fully analyze the effects and interaction effects of factors, and explore the optimal process space
DOE reduces process research time, comprehensively understands and analyzes processes

Mr. Xu 19025438700

Business Cooperation:
Dr. Xu 19025438700 WeChat:xiaoboo365
Virus Vector:
Manager Shi 15391503730 WeChat:shixiaotai611
Nucleic Acid Products:
Manager Ni 13437112357 WeChat:go-ahead-no-back
Protein Products:
Manager Zhou 18771147489 WeChat:huhu_ai_junjun
Genetic Products:
Manager Wang 13067989761 WeChat:wry12121
Instrument and Equipment:
Manager Ni 13437112357 WeChat:go-ahead-no-back
Test Kit:
Manager Liu 15207178732 WeChat:Q15207178732

xuxiaobo@binhui-bio.com


